Media
HKUMed unveils mechanisms of Chchd10 regulation in adipose tissue homeostasis, key to effective obesity management
17 Mar 2025
Obesity is a global health concern closely linked to a range of metabolic diseases, such as type 2 diabetes and cardiovascular diseases. A research team from the Department of Pharmacology and Pharmacy, LKS Faculty of Medicine at the University of Hong Kong (HKUMed), identified the mitochondria protein Chchd10 as a novel regulator of adipose tissue homeostasis, offering new insights into the management of obesity and its related metabolic disorders. The findings were published in Advanced Science [link to the publication].
Background
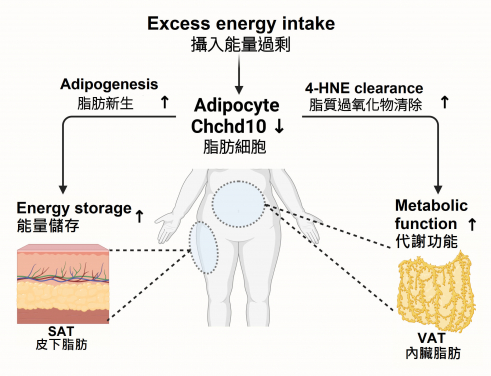
The health of adipose tissue is crucial for maintaining the body’s energy balance and metabolic health. In response to excess energy intake, adipose tissue undergoes remodelling by increasing the number and size of fat cells. However, an imbalance in this remodelling process can lead to obesity and related metabolic disorders. The accumulation of fat in adipose depots in different anatomical locations can lead to distinct metabolic outcomes. Excessive visceral adipose tissue is a risk factor for metabolic complications, but lipid storage in subcutaneous adipose tissue is considered less harmful. Understanding the molecular mechanisms that govern adipose tissue homeostasis is therefore essential for developing effective interventions.
Research methods and findings
The HKUMed research team investigated the role of Chchd10 in adipose tissue remodelling using high-fat diet (HFD)-induced wild-type and adipose tissue-specific Chchd10 (AT-Chchd10)- deficient mice. The study found that in HFD-induced mice, the levels of Chchd10 were significantly reduced in white adipose tissue, which is responsible for energy storage and metabolism regulation. This reduction accelerated subcutaneous fat formation in the mice, allowing them to store more energy during short-term, high-fat diet periods. Conversely, the absence of Chchd10 resulted in elevated levels of GSTA4, a protein found in visceral fat tissue that is essential for helping clear harmful lipid peroxidation products, preventing protein carbonylation and cellular dysfunction after long-term, high-fat diet consumption. The study highlighted that the downregulation of Chchd10 activates the NRF2 signalling pathway, which promotes subcutaneous fat formation and GSTA4 expression.
Significance of the study
The findings demonstrate that Chchd10 is a novel regulatory factor that maintains stability and balance in adipose tissue and that downregulation of Chchd10 enhances subcutaneous fat formation and regulates antioxidant capacity mainly in visceral adipose tissue, thereby reducing the risk of obesity and metabolic disorders induced by diet. Additionally, in mice undergoing weight gain from a high-fat diet, knocking out Chchd10 in adipose tissue significantly reduced the increase in visceral fat mass, suggesting that regulating Chchd10 levels could be a potential therapeutic strategy for treating obesity.
Professor Ruby Hoo Lai-chong, Associate Professor in the Department of Pharmacology and Pharmacy, HKUMed, said, ‘This study provides valuable insights into the molecular pathways involved in regulating adipose tissue homeostasis and their implications for obesity management. By targeting Chchd10 and its associated pathways, new therapeutic strategies can be developed to combat diet-induced obesity and improve metabolic health. The research underscores the potential interventions targeting metabolic dysfunctions in specific adipose depots.’.
Another study: Discovery of novel immune cells in type 1 diabetes
In another study, Professor Hoo’s research team uncovered the role of tissue-resident memory T (TRM) cells in the development of type 1 diabetes. The study explored how TRM cells, through the fatty acid-binding protein 4 (FABP4) and the inflammatory chemokine CXCL10, orchestrate the recruitment of cytotoxic T cells to pancreatic islets, thereby playing a significant role in the onset of type 1 diabetes.
The research demonstrated that genetic deletion of FABP4 or depletion of TRM cells in non-obese diabetic mice led to reduced cytotoxic T cell recruitment, delayed diabetes onset, and suppressed chemokine CXCL10 production. These findings suggest that targeting FABP4 could offer a promising therapeutic strategy for managing type 1 diabetes by mitigating immune-mediated damage to pancreatic cells. The findings were published in Advanced Science [link to the publication].
About the research team
The studies were led by Professor Ruby Hoo Lai-chong, Associate Professor in the Department of Pharmacology and Pharmacy, HKUMed. Other research team members included Dr Wu Xiaoping and Dr Zhang Zixuan from the same department; and Professor Xu Aimin from the Department of Medicine, School of Clinical Medicine, and State Key Laboratory of Pharmaceutical Biotechnology, HKUMed.
Acknowledgments
The two studies were supported by the Health and Medical Research Fund, the Guangdong Basic and Applied Basic Research Fund, the Young Scientists Fund of the National Natural Science Foundation of China, the Area of Excellence Scheme and the Collaborative Research Fund of the Hong Kong Research Grants Council.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).